Development Pipeline
Aisa is enrolling patients with Secondary Raynauds, primarily due to Scleroderma in Australia in the RECONNOITER-1 study. This is a two-part study, with the first part (planned 36 patients) being dose-finding and parallel design arms and the second part (planned 40 patients) being a double-blind randomized crossover design where each patient is dosed with either placebo or active treatment separated by a washout period. The first part of the study has been completed, and the second part is nearly complete.
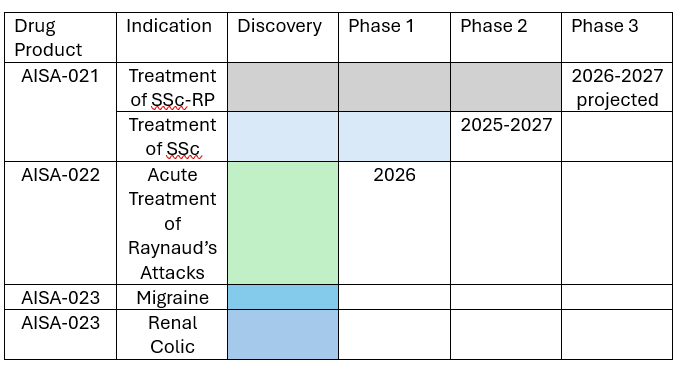
Aisa is also planning several other development programs and has evaluated the biologic actions of AISA-021 in preclinical studies. These activities are ongoing and the NIH is studying Aisa’s AISA-021 as well in its Preclinical Screening for Pain Platform studies. To date, this program has demonstrated the drug to have no potential for abuse or addiction and predictable and reproducible pharmacokinetics. The following diagram illustrates all of Aisa’s development pipeline and plans:
AISA 021 and Development Pipeline